Today’s blog is written by guest bloggers Hanson E1,2, Gonzalez Tirado D1, Bass B1, Werking J3, Lagace R3 and Ballantyne J1,2
1National Center for Forensic Science, University of Central Florida, Orlando, FL USA
2Department of Chemistry, University of Central Florida, Orlando, FL USA
3ThermoFisher Scientific, San Francisco, California, USA
Rapid DNA fully integrates sample analysis workflow from extraction to capillary electrophoresis, generating autosomal STR profiles in as little as 90 minutes without the need for a DNA laboratory. The FBI allows DNA profiles developed from reference samples on rapid DNA instruments to be uploaded to CODIS, but not from crime scene samples. Reference samples collected from arrestees at some booking stations are being automatically uploaded and searched against CODIS unsolved crime scene profiles. This practice is expanding nationwide because of its ability to link arrestees to unrelated crimes while he/she is still in custody.
Although the FBI does not currently allow DNA profiles generated from crime scene samples on rapid DNA instruments to be uploaded to CODIS, they are working with vendors to enable this capability in early 2025. In the meantime, many law enforcement agencies have been using rapid DNA outside of CODIS to substantially impact criminal investigations, human trafficking, and the identification of human remains. While most of this work has been done with blood and saliva cases, rapid DNA is rarely used for sexual assault cases because rapid DNA instruments do not perform differential extractions. We sought to develop an off-instrument differential extraction method that was compatible with non-technical users and rapid DNA instruments to enable law enforcement to take advantage of the speed of rapid DNA in sexual assault cases.
The goal of the current work was to develop simplified DE methods for the RapidHIT™ ID system for use with semen-containing evidence found in sexual assault investigations. The methods developed are designed to be used for investigative leads in a laboratory or potentially in point-of-collection use environments. The outcome is a simple workflow that utilizes a 1-hour differential lysis (using a stain extraction buffer and proteinase K) to preferentially lyse epithelial cells leaving sperm cells intact. After a few brief washes, the epithelial cell fraction is separated, and the remaining sperm pellet can then be collected with a sterile micro flocked swab and run using the RapidINTEL™ cartridges on the RapidHIT™ ID system. No additional processing of the sperm pellet prior to being placed on the instrument is needed.
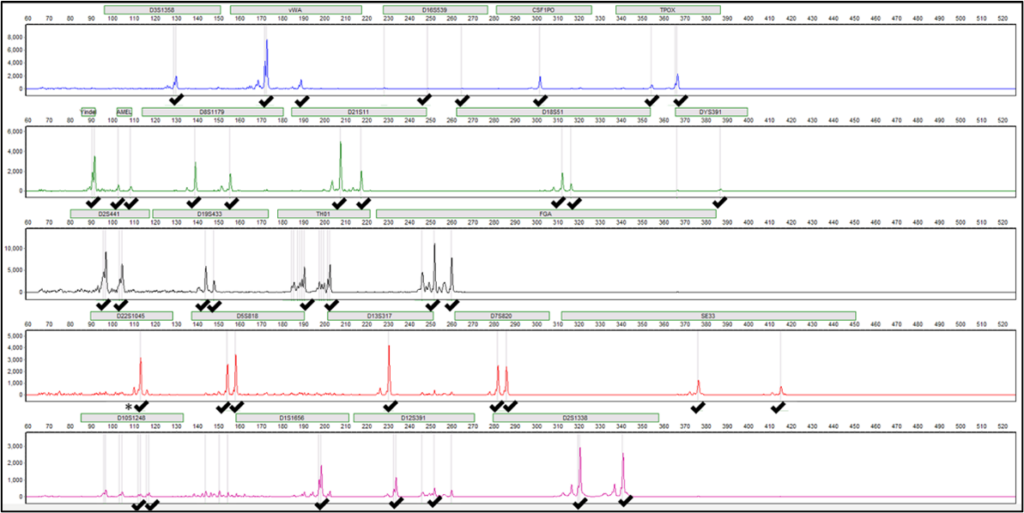
Using this simplified DE method, single source male DNA profiles can be obtained from as little as 1 µl of semen, with the full sensitivity of the method still being evaluated. Mock mixtures using both buccal and vaginal epithelial cells were evaluated representing possible casework scenarios of vaginal and oral assaults. Representative profiles from an epithelial-semen admixed sample with 5 µl and 1 µl are shown in Figure 1. A full male profile, with no residual female alleles detected, was obtained for the 5 µl semen admixture. For the 1 µl semen admixture, a probative partial male profile (33/40 alleles, ~83%) was obtained with only three alleles unique to the female donor observed. Lysis times of less than 1 hour were tested and were successful with as little as a 5-minute lysis. However, allele recovery with 1 µl semen samples was not as successful with the reduced lysis time and therefore it was kept at 1 hour.

Successful results using the developed method were also obtained from volunteer donated 12- and 24-hr cervicovaginal post coital samples. An example of a single source male profile obtained from a cervicovaginal sample collected 24 hours after intercourse is shown in Figure 2, with only one male allele drop out (36/37, ~97%) and no residual female alleles observed.
Rapid DNA fully integrates sample analysis workflow from extraction to capillary electrophoresis in a single reaction. Despite this being an integrated single reaction, there is remaining sample in several steps of this process that can be retained and/or put through standard laboratory workflows if needed. This protocol is intended for use in cases where two swabs are collected, one being used in the simplified DE RapidHIT™ protocol and the other swab retained for standard analysis. Within the RapidHIT™ processed sample, the micro flocked swab used to collect the sperm pellet can be removed from the cartridge after analysis and run a second time, as some biological material will still be present on the swab. Alternatively, that swab could be sent through standard DE workflows. The original cotton swab substrate that the sexual assault sample remaining after sperm pelleting can also be run on the RapidHIT™ or with standard DE workflows. Lastly, the original sample lysate tube can be swabbed or directly extracted from to recover any residual male DNA that may be remaining in the tube. These fail safes are important to note to prevent any concerns regarding loss of critical evidence and to permit standard workflows to be used for the same sample to confirm any findings.
Future work will be focused on transition of this method to the new RapidINTEL™Plus cartridges and to further optimize this method including an evaluation of additional post coital samples including extended interval samples (3-5 days after intercourse). We are hopeful that these studies can provide necessary preliminary data to demonstrate the usefulness of a rapid DNA approach to improve the timeliness of sexual assault evidence analysis permitting the development of an investigative lead early on in an investigation.
WOULD YOU LIKE TO SEE MORE ARTICLES LIKE THIS? SUBSCRIBE TO THE ISHI BLOG BELOW!
SUBSCRIBE NOW!


