Today’s sponsored guest blog is written by Promega.
This article summarizes the developmental validation of the PowerPlex® 35GY System, an 8-dye STR multiplex for human identification applications. This system is optimized for use with Spectrum CE Systems and aims to amplify 35 loci, including all CODIS and ESS markers, Amelogenin, and DYS391 for gender determination. The study aims to validate the system’s reliability and robustness for forensic DNA typing applications.

Introduction
The PowerPlex® 35GY System is designed to enhance the capabilities of forensic DNA typing by offering a comprehensive set of 35 loci employing an 8-dye technology, allowing the inclusion of more loci in a single amplification, which increases the chances of successful analysis of challenging samples.
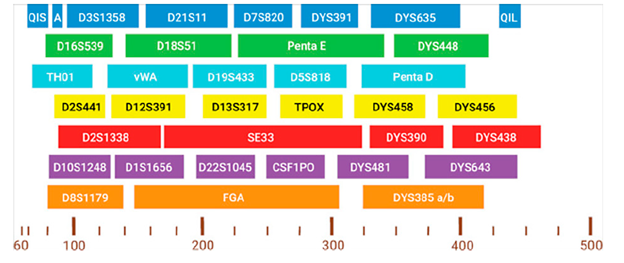
The PowerPlex® 35GY System amplifies the autosomal loci in the CODIS and ESS core set, including CSF1PO, FGA, TH01, TPOX, vWA, D1S1656, D2S1338, D2S441, D3S1358, D5S818, D7S820, D8S1179, D10S1248, D12S391, D13S317, D16S539, D18S51, D19S433, D21S11, and D22S1045, along with Amelogenin and DYS391 for gender determination and Penta D, Penta E and SE33 for increased discrimination. and database searching. Ten additional nonrapidly mutating Y-STR loci (DYS635, DYS448, DYS458, DYS456, DYS390, DYS438, DYS481, DYS643, DYS385a/b) are included for familial searching and to assist with the interpretation of sexual assault evidence profiles. The system includes two Quality Indicators to enhance the analysis and interpretation of STR data. The Quality Indicator Small (QIS) and the Quality Indicator Large (QIL) cover the lower and higher base pair range of amplification products. The quality peaks aid in assessment of the overall success of the amplification reaction by helping to differentiate between degraded and inhibited samples. The incorporation of smaller amplicon sizes, facilitated by the 8-dye channels, enhances the system’s performance with degraded samples.
The developmental validation study was conducted following the guidelines set by the Scientific Working Group on DNA Analysis Methods (SWGDAM) and was a collaborative effort involving multiple laboratories, including the New Hampshire State Police Forensic Laboratory (NHSPFL), the Federal Bureau of Investigation (FBI), the Forensic Genomics Innovation Hub (FGIH), the Servicio de Criminalística de la Guardia Civil (SECRIM), the National Institute of Standards and Technology (NIST), and Promega Corporation. This multi-institutional collaboration provided a robust dataset and comprehensive evaluation of the system’s performance.
The developmental validation (DV) data presented in the paper is a composite of results generated by all participating laboratories. This approach ensures a broad and representative assessment of the PowerPlex® 35GY System’s capabilities and reliability across different settings and conditions.
Highlights from the DV Study:
Following amplification of DNA from purified and direct amplification substrates under different conditions elaborated in the methods section of the paper, the DNA fragment detection was performed on the Spectrum CE System, 8-Capillary instrument following calibrations using the PowerPlex 8C Matrix Standard. Files generated by the Spectrum CE Systems (.promega format) were analyzed with GeneMarker_ HID Software for Spectrum CE Systems (v3.2.0) using the appropriate panel, bin, and stutter values for analysis. Baseline noise and threshold analyses studies were performed with multiple instruments to determine Analytical threshold (AT) values for extracted DNA and direct amplification samples.
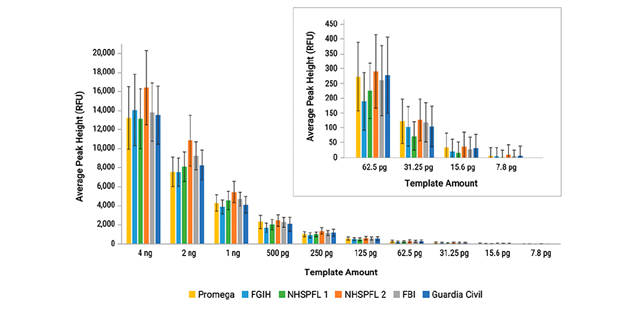
Sensitivity studies, which tested DNA from two donors with input concentrations ranging from 4 ng to 7.8 pg to determine the system’s dynamic range, showed that the system successfully detected all alleles at concentrations as low as 125 pg, with a decrease in the percentage of alleles called below this concentration.
Specificity of primers for human DNA was assessed by testing cross-reactivity with non-human DNA, including microorganisms, vertebrates, and non-human primates. Results show that no amplification products were detected in the size range of 60 to 500 nucleotides for any microbial species tested. The paper contains additional details on the artifact peaks produced from some non-primate vertebrates, like chicken, horse, and pig. Non-human primate species yielded profile peaks due to genetic similarities but were also distinguishable from human DNA.
Stability studies included the kit’s performance in the presence of inhibitors (EDTA, hematin, humic acid, and tannic acid) and degraded DNA (DNA samples were exposed to UV radiation). Degraded DNA samples exposed to increasing UV radiation showed a decrease in average peak height and percent profile, with preferential loss of high-molecular-weight loci. The Quality Indicator ratios remained at or above 1.0, aiding in distinguishing degraded samples from inhibited ones. The QIL peak height was reduced when a sample contained higher levels of inhibition, highlighting the utility of PowerPlex 35GY Quality Indicators in distinguishing degraded samples from inhibited samples. Below is an example of PowerPlex 35GY performance with degraded samples.

Case-Type Samples and Mixtures:
A variety of forensic sample types, including blood, bones, saliva, and mixed samples, were tested by multiple labs to demonstrate the system’s versatility. Profiles generated were consistent with the expected source of DNA and the quantity. When partial profiles were generated, genotype information was still attained, particularly at loci approximately 250 bp or less. Many partial profiles exhibited signs of degradation with sloping pattern, loss of high molecular-weight loci, and balanced Quality Indicators. A few samples exhibited inhibition depicted by a sloping pattern with a reduced or complete loss of QIL peak.
Two-person and three-person mixtures were evaluated to determine the system’s ability to resolve minor contributor alleles. In two-person mixtures, minor contributor alleles were resolved in the following ratios: 5:1, 2:1, 1:1, 1:2, and 1:5. In three-person mixtures, 98 % of total alleles were detected in the 1:1:10 ratio, and 89 % in the 1:1:20 ratio.
Precision and Accuracy
- Allelic Ladder Precision: Sizing precision was evaluated using 24 PowerPlex® 35GY allelic ladders injected on five different Spectrum CE Systems. Standard deviations were ≤0.09 nt, and size ranges were ≤0.30 nt.
- Reproducibility and Repeatability: Forty-eight 2800M samples showed 100% genotype concordance across instruments, matching the profile in the PowerPlex® 35GY technical manual.
- Concordance: A large concordance study comparing PowerPlex® 35GY genotypes with other STR systems showed a 99.98% concordance rate, with nine discordant calls out of 38,999 alleles compared.
PCR-Based Studies:
- Thermal cyclers: The system’s performance was evaluated on various thermal cycler models and consistent performance was observed, with high peak heights and complete allele calls at different DNA concentrations. Average peak heights for 1 ng of DNA ranged from 4,000 RFU to 5,000 RFU, demonstrating reliable amplification across different cyclers.
- Cycle Number: Increasing the cycle number resulted in higher average peak heights for both 1 ng and 200 pg samples. Full profiles were obtained at 28, 29, and 30 cycles for 1 ng samples.
- Annealing Temperature: Consistent performance was observed across annealing temperatures of 58°C, 60°C, and 62°C, with slight variations in peak heights for some loci.
- Reaction Volume: Amplification reactions conducted at 25 μL and 12.5 μL volumes showed higher peak heights for the smaller volume when DNA mass was fixed.
- Reaction Components: Varying primer pair and master mix concentrations resulted in consistent overall average peak heights, demonstrating the system’s flexibility with different reagent concentrations.
Conclusion:
The PowerPlex® 35GY System provides reliable and robust performance for forensic DNA typing, with high sensitivity, specificity, and tolerance to inhibitors and degraded samples. Its ability to handle a variety of sample types and conditions makes it a valuable tool for forensic laboratories, offering significant improvements in the analysis of challenging samples. The system’s precision, accuracy, and flexibility with different thermal cycler models and reaction conditions further enhance its utility in forensic applications.
This summary provides an overview of the key findings and features identified during the developmental validation of PowerPlex® 35GY System. For detailed experimental data, figures, and tables, readers are encouraged to refer to the original paper.

